20 October, 2025
What Is Battery Self-Discharge and How to Calculate It
Battery self-discharge is a critical phenomenon in electrochemical energy storage, referring to the natural capacity loss that occurs when a battery is in an open-circuit state over time. Understanding the principles, influencing factors, and calculation methods of self-discharge is essential for effective battery storage management, lifetime prediction, and performance optimization.
This article explains the mechanism of self-discharge, the rate differences across various battery chemistries, calculation methods, and practical strategies to minimize its impact—helping engineers and users better maintain and extend battery life.
What Is Battery Self-Discharge
Battery self-discharge refers to the gradual loss of stored capacity over time when the battery is in an open-circuit state. It consists of both physical and chemical self-discharge and is an inherent property of all batteries. Understanding this process is vital for evaluating battery life, storage performance, and overall reliability.
How Does Self-Discharge Happen
Self-discharge occurs due to internal chemical reactions similar to those in closed-circuit discharge, even when the battery is not in use. High temperatures accelerate these reactions, leading to faster capacity loss, while lower temperatures help slow them down. Over time, a passive layer forms on the electrode surface, acting as a protective film that can partially reduce self-discharge.
Main causes of self-discharge include:
- Electrochemical stability: Deviation from ideal equilibrium conditions causes energy loss as heat.
- Material degradation: Over time, internal materials age and trigger side reactions that consume stored energy.
- Ionic transport efficiency: Poor ion mobility within the electrolyte increases the self-discharge rate.
The quality of internal components also plays a crucial role. Impurities in the electrolyte or electrode materials can accelerate self-discharge, and defects in the separator may lead to micro short-circuits, further increasing energy loss.
Self-Discharge Rates in Different Battery Chemistries
Although self-discharge is an inherent characteristic of all batteries, its rate varies across chemistries. For example, lithium-ion batteries exhibit relatively low self-discharge (about 2–3% per month), while nickel-metal hydride (NiMH) batteries can reach 10–30% per month.
Main causes of self-discharge include:
| Battery Chemistry | Rechargeable | Typical Self-Discharge / Shelf Life |
| Lithium Metal | No | ~10 years shelf life |
| Alkaline | No | ~5 years shelf life |
| Zinc–Carbon | No | 2–3 years shelf life |
| Thionyl Chloride | No | ~1% per year |
| Lithium-ion | Yes | 2–4% per month |
| Lithium Polymer | Yes | ~5% per month |
| Low Self-Discharge NiMH | Yes | ~0.25% per month |
| Lead–acid | Yes | 4–6% per month |
| Nickel–Cadmium | Yes | 15–20% per month |
| Conventional NiMH | Yes | ~30% per month |
How to Calculate Battery Self-Discharge
Accurate calculation of the self-discharge rate is crucial for assessing shelf life, storage performance, and reliability.
Formula for Calculating Self-Discharge
The standard formula is: Self-Discharge Rate(%) = (C0− Ct) /C0 × 100
where:
C0: Initial capacity before storage (Ah or mAh)
Ct: Remaining capacity after storage period t
t: Storage time (days, weeks, or months)
This formula expresses percentage capacity loss relative to the initial capacity.
Step-by-Step Calculation Example
Example: A 100 Ah lithium-ion battery is stored at 25°C for one month. After testing, its remaining capacity is 96 Ah.
Self-Discharge Rate=[(100−96)/100] × 100=4%
Thus, the battery lost approximately 4% of its capacity per month under these storage conditions.
Testing Conditions and Best Practices
To ensure accurate and comparable results, follow these practices:
- Fully charge the battery before storage.
- Store under constant temperature (typically 20–25 °C).
- Measure capacity after storage using the same discharge rate and cutoff voltage.
- Record storage duration precisely.
- For reliability studies, repeat tests under different temperatures.
Factors Affecting Self-Discharge
The rate of battery self-discharge is influenced by multiple factors, including battery chemistry, ambient temperature, state of charge (SOC), storage time, aging, impurities, and cycling history.
Battery Type
Cathode Materials: High-nickel cathode materials typically exhibit higher self-discharge rates than lithium iron phosphate (LFP). Due to its stable olivine structure, LFP offers much lower self-discharge and better long-term stability.
Anode Materials: Silicon-carbon anodes experience significant volume changes during cycling, leading to unstable SEI (Solid Electrolyte Interphase) layers and higher self-discharge rates compared with graphite anodes.
Temperature
Temperature is one of the most critical factors affecting self-discharge. Generally, chemical reaction rates approximately double with every 10°C rise in temperature. Elevated temperatures significantly accelerate all parasitic side reactions, leading to a sharp increase in self-discharge rate.
State of Charge
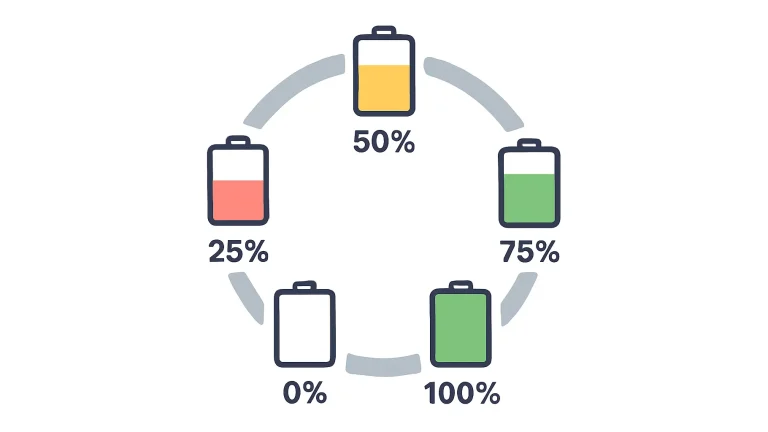
A higher SOC corresponds to higher electrode potentials, which increases the reaction driving force between electrode materials and electrolyte. As a result, the self-discharge rate rises with increasing SOC. For long-term storage, maintaining the battery around 50% SOC is generally recommended.
Time
Self-discharge is a continuous process. The longer the battery remains idle, the greater the cumulative capacity loss.
Battery Aging and Impurities
As batteries age, both electrodes and electrolytes undergo physical and chemical transformations that accelerate self-discharge.
Aged batteries often exhibit microcracks, increased internal resistance, and unstable surface films. These defects create small leakage paths or enable ongoing side reactions even under open-circuit conditions, resulting in gradual energy loss.
Charge/Discharge History and Cycle Count
The charging and discharging history of a battery greatly affects its self-discharge behavior. Frequent deep cycles, overcharging, or over-discharging cause irreversible chemical changes in electrode materials, forming unstable surface regions and localized stress points, which increase internal leakage currents.
Batteries that have undergone extensive cycling tend to exhibit higher self-discharge rates, as the SEI protective layer becomes thinner, uneven, or damaged over time, losing its ability to suppress parasitic reactions.
How to Reduce or Manage Self-Discharge
Store in a Cool, Dry Environment
Store batteries in a cool and dry place between 15°C and 25°C, avoiding freezing temperatures. High temperatures accelerate self-discharge, while extremely low temperatures may reduce performance. Typically, for every 10°C increase, the self-discharge rate doubles or triples.
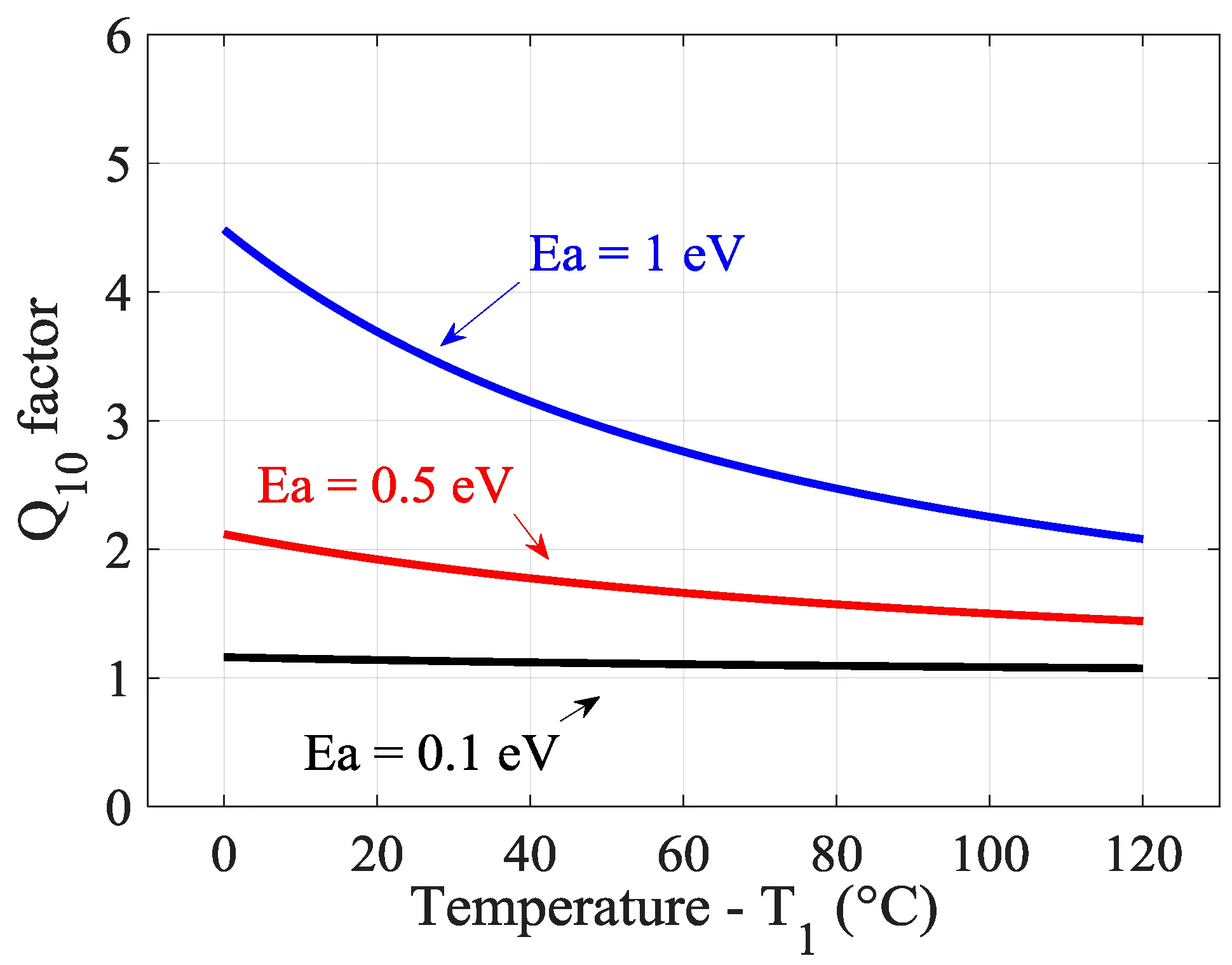
For example, at 25°C the self-discharge rate is about 2% per month, while at 55°C it can rise to 8% per month.
Perform Regular Maintenance Charging
For batteries left unused for extended periods, periodic maintenance is essential. Check the charge level every 3–6 months and perform a full charge-discharge cycle to maintain activity and prevent irreversible damage caused by deep discharge.
Maintain an Optimal State of Charge
For long-term storage, maintain a 40–60% SOC. Avoid storing batteries at full charge or completely discharged states. Full charge accelerates electrolyte–cathode reactions, while complete discharge may cause anode degradation.
Select Low Self-Discharge Battery Chemistries
Compared with other chemistries, lithium-based batteries offer the best balance between energy retention and cost, typically exhibiting 2%–3% self-discharge per month.
Use Battery Management Systems
Implement a Battery Management System (BMS) to monitor real-time parameters such as voltage and temperature. The BMS can detect anomalies early and trigger protective actions, preventing accelerated degradation and energy loss.
Conclusion
Battery self-discharge is a continuous and inevitable process influenced by factors such as chemistry type, temperature, state of charge, aging, and cycling history. By accurately measuring self-discharge, applying optimal storage and maintenance practices, and utilizing low self-discharge chemistries and smart monitoring systems, energy loss can be minimized, and battery life extended.
For engineers and end-users alike, monitoring and managing self-discharge is not only key to enhancing battery performance but also critical for ensuring device reliability and optimizing energy storage system design.
share